Corvion: Developing New LVAD Technology with Potential to Transform Heart Failure Treatment
Corvion is a medical technology company working to develop an innovative Left Ventricular Assist Device (LVAD) for patients with end-stage heart failure. This article aims to provide an overview of Corvion's efforts and potential market opportunities based on information provided by the company. It's important to note that Corvion's device is still in development and has not yet been approved for patient sale or use.
Understanding LVADs and Current Limitations
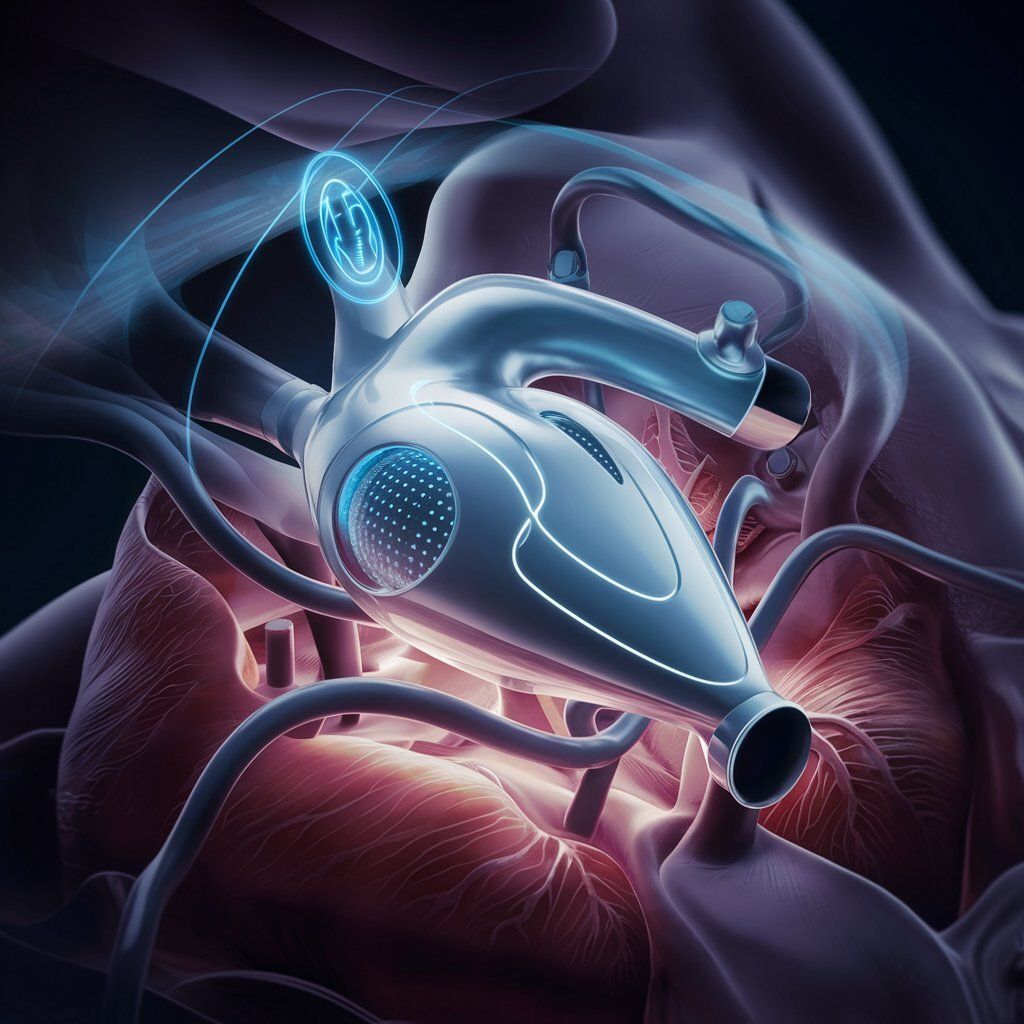
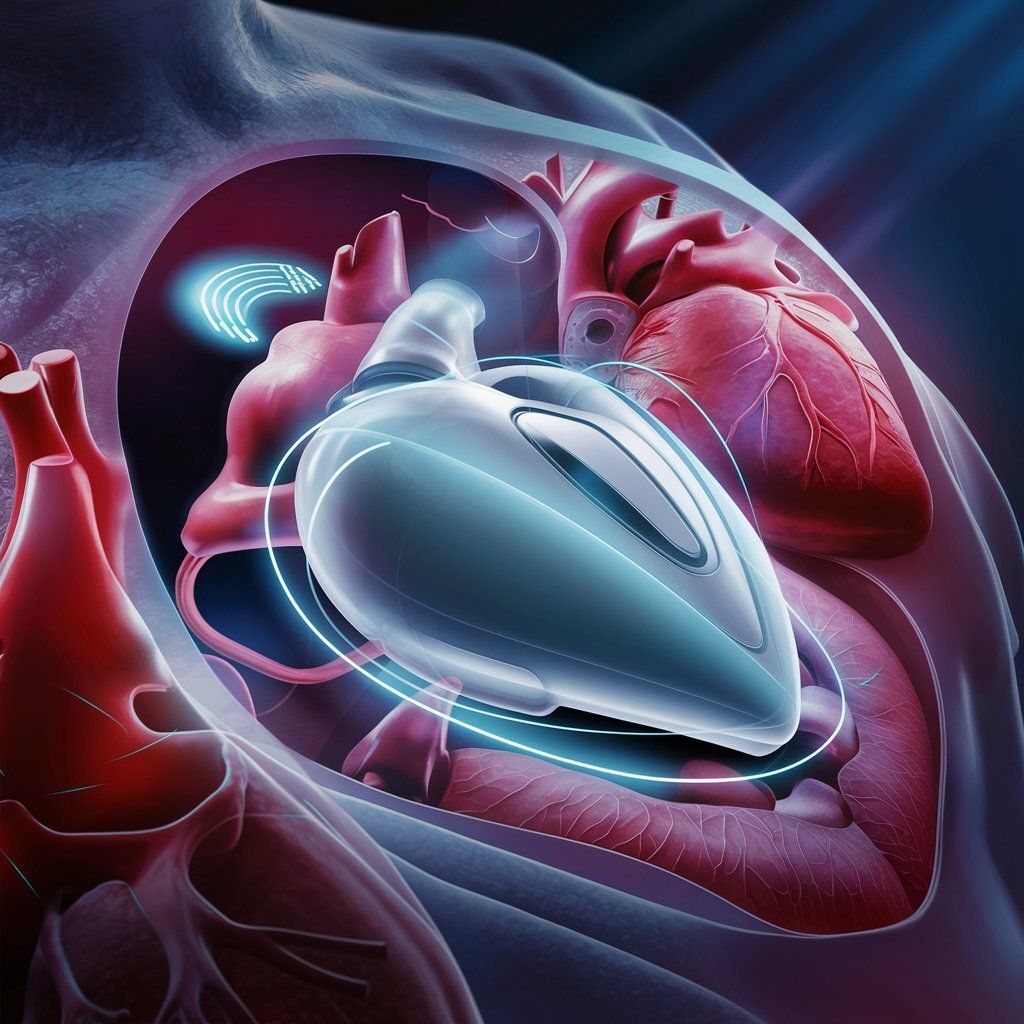
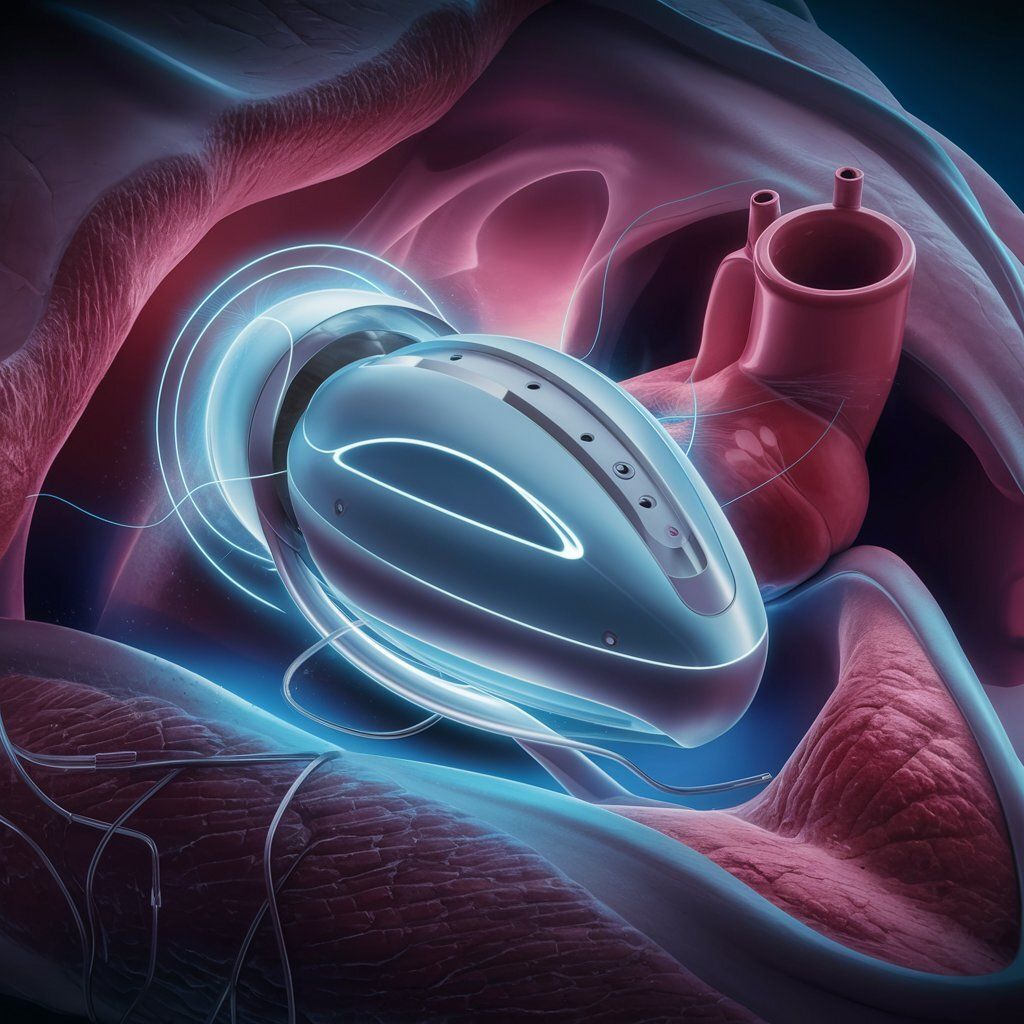
An LVAD is a mechanical pump that assists the heart's left ventricle in pumping blood. While current LVADs have helped many patients, they face challenges that Corvion aims to address:
Infection risk associated with external components
Limited patient mobility due to external equipment
Restrictions on bathing and swimming
Potential psychological impact of visible external components
These factors may contribute to the limited adoption of current LVAD technology.
Corvion's Approach to LVAD Technology
Corvion is working to develop an LVAD with the following potential features:
Fully implantable design
Wireless charging capability
Aim for improved efficiency compared to existing devices
Goal of enhancing patient mobility and quality of life
It's important to note that these features are still in development, and their effectiveness has not been proven in human trials.
Leadership Team
Corvion's development efforts are led by a team with experience in medical device innovation and business:
Greg Aber (Founder and CEO/CTO): Background in NASA engineering and medical device development
Dr. Eric Rose (Board Member): Experienced heart surgeon
Eugene Glover (Principal Investor and Board Member): Co-founder of Mentor Corporation
Sean Morris (Board Member): Experience in medical device startups
Market Opportunity
Corvion believes there may be potential for significant market expansion in LVAD technology. While the current LVAD market is estimated at around $1 billion annually, some industry analysts suggest the total addressable market could be larger if adoption increases. However, market size projections are inherently uncertain and subject to numerous factors.
Corvion's Progress to Date
Corvion reports the following developments:
Raised $22 million in funding
Holds patents related to their LVAD technology
Received FDA Breakthrough Device Designation
Awarded an NIH Phase 1 SBIR Grant
Conducted preclinical animal studies
Future Plans
Corvion's stated goals include:
Pursuing First-In-Human trials
Conducting Early Feasibility Studies
Seeking FDA approval
Developing various LVAD models for different patient needs
These plans are subject to change and dependent on various factors, including clinical trial results and regulatory approvals.
Potential Impact and Considerations
If successfully developed and approved, Corvion's technology could potentially:
Provide a new treatment option for some heart failure patients
Aim to improve quality of life for LVAD recipients
Potentially reduce certain healthcare costs associated with current LVAD therapy
However, it's crucial to understand that medical device development involves significant risks and uncertainties. Success is not guaranteed, and numerous challenges must be overcome before a device can reach the market.
Challenges and Risks
Investors and interested parties should be aware of the following challenges and risks:
Regulatory hurdles in gaining FDA approval
Uncertainty of clinical trial outcomes
Complexities in manufacturing and scaling production
Potential difficulties in market adoption
Possible competition from other companies
Uncertainties in securing insurance reimbursement
Conclusion
Corvion is developing new LVAD technology to address limitations in current devices. While their approach shows promise, it's important to remember that the device is still developing and faces numerous hurdles before potential commercialization. The company's progress should be monitored closely as it works towards human trials and seeks regulatory approvals.